TrialStat eClinical Suite - Powering Simple to Complex Clinical Trials
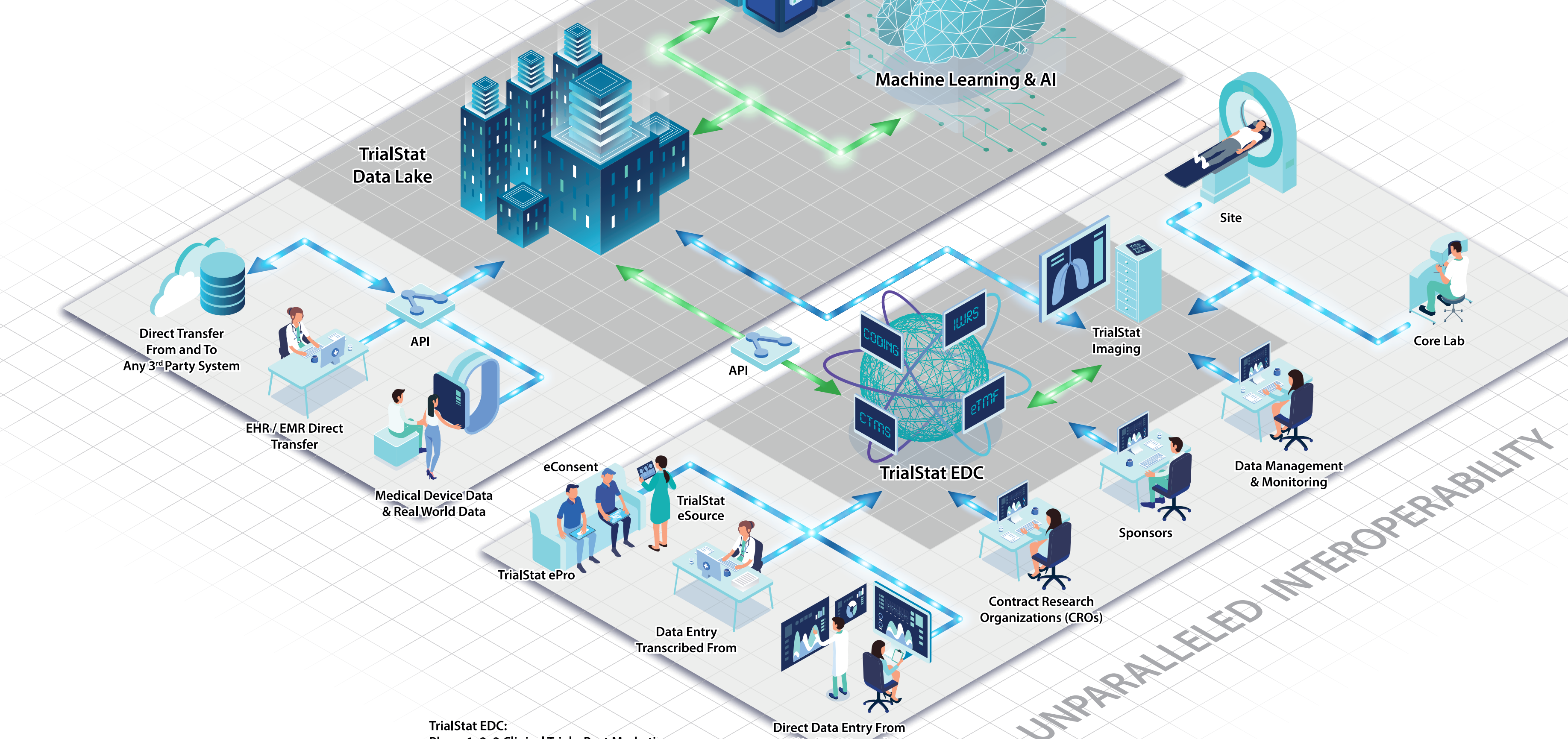
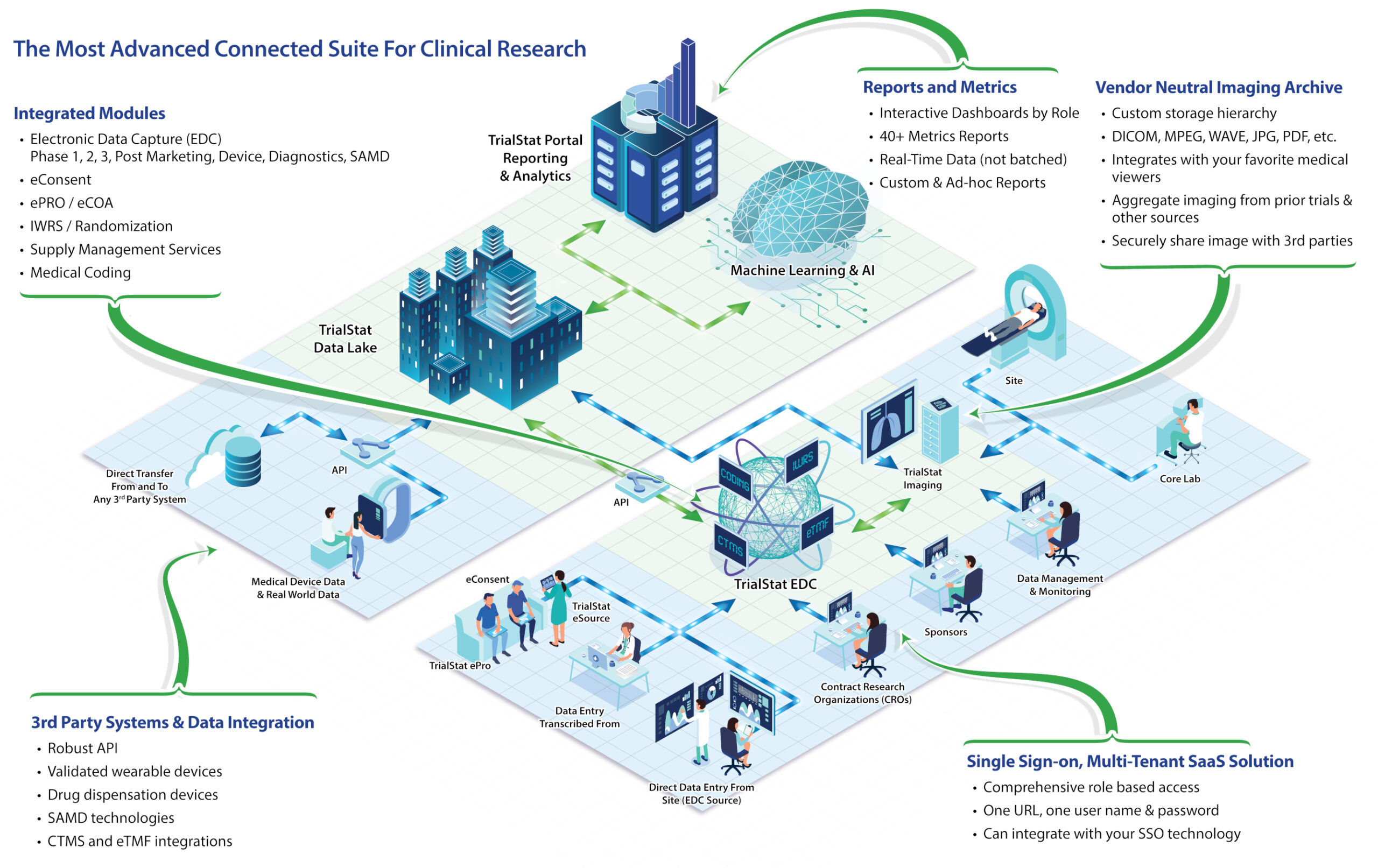
TrialStat® delivers data solutions on-demand for pharmaceutical, biotechnology, medical device companies, Contract Research Organizations and Data Management companies. Our TrialStat eClinical Suite™ consists of EDC, Reporting Portal and Vendor Neutral Imaging Archive. We stand alone in the industry with such an all-compassing suite of products for conducting clinical trials across all therapeutic areas.
TrialStat EDC
TrialStat EDC is a scalable, hosted, Electronic Data Capture Suite for pilot / proof of concept studies, Phase I, II, III and IV studies. In addition, TrialStat EDC is an ideal choice for the unique requirements of Medical Device and Diagnostic studies. TrialStat EDC uses the latest technology, allowing all users complete system access via any web browser or mobile device.
Explore TrialStat EDC
Visit the TrialStat EDC page to explore additional information and download the TrialStat EDC Overview.
TrialStat eConsent
Accelerate trial enrolment and maximize protocol compliance by leveraging TrialStat's comprehensive and immersive eConsent experience. Configurable workflows, support for witnesses, assent, re-consent, and the ability for patients to revoke consent.
TrialStat's eConsent is fully integrated with TrialStat's EDC or it can be used with other EDC platforms!
Explore TrialStat eConsent
Contact us today to find out more about the eConsent module and see how it works!
Request Your Demo Today!
From rapid database build through database lock, we deliver consistent quality on-time and on-budget. Ready to upgrade your eClinical toolkit?
See What Our Clients Are Saying About TrialStat
“Plus Therapeutics found a reliable, scalable partner in TrialStat Solutions as we transition from manual processes. Their unified eClinical suite simplifies data collection, provides real-time insights, and offers modular features and functions that will allow us to expand into their cost-effective system as we grow. Their dedication to innovation and customer satisfaction is crucial to our success and we highly recommend them.”
- Norman LaFrance, Chief Medical Officer, SVP, Plus Therapeutics
“Dear Heather and Jeff,
I hope this email finds you both well. On behalf of the Plus Therapeutics team, I am grateful to both of you for hosting the exceptional onboarding EDC training for the Plus Therapeutics team and our site staff yesterday. Your expertise and dedication truly shone through, and it was a fantastic learning experience for all of us.”
-Rosemary Afful, Senior Clinical Research Associate, Plus Therapeutics
“If you ask the TrialStat team a question, the answer is never “No”, but “let’s see how we can accomplish that!”
- Colin Miller, CEO, The Bracken Group
“The knowledgeable team at TrialStat worked with us to develop a validated custom web application, integrated with both EDC and our internal systems, that streamlines workflow and maximizes our team’s productivity. We can collect and manage trial data in a more timely and efficient manner now, which makes our clients happy too.”
- Judi Hall, VP Clinical Research at Alimentiv
"TrialStat surpassed our expectations with their exceptional customer service and intuitive EDC Platform. Their personalized support and expert guidance made navigating the critical tasks of customizing the eCRFs and data migrations in our complex studies, remarkably straightforward and easier! Compared to other platforms we considered, TrialStat's tailored approach and unwavering timely assistance have been instrumental in the successful execution and conduct of our trials."
- Conroy Campbell, Director of IT, Plus Therapeutics
Explore TrialStat's Comprehensive eClinical Suite
See TrialStat In Action
Request Your Personalized Demo. Our Clinical Team will organize a tailored demonstration of TrialStat based on your specific study requirements. You'll see first hand how TrialStats unified features, blazing speed, configurable features and comprehensive functionality will help you run more efficient and cost effective studies.
Premium Modules & Custom Development Services
A Fully Unified eClinical Suite Offering EDC functionality with eConsent, Randomization and IWRS, ePRO, Medical Coding, and Vendor Neutral Imaging Archive in a single platform.
Custom Development
TrialStat is unique in the industry, providing validated custom development services. As a matter of practice, we regularly include new features based on Sponsor requirements. Our expert Software Architects, Software Developers and Compliance Experts provide complete custom development services to meet your unique requirements while ensuring compliance with all relevant regulatory requirements such as 21 CFR Part 11, HIPAA and Privacy Shield.
Tired of Hearing "No" All The Time?
Do you have requirements for your studies which other EDC platforms can't support?
Don't worry, at TrialStat we've developed our technology specifically for you. Our Expert Clinical Consultants and Software Developers will work with you to implement the features you need within our Validated eClinical Suite.
We're Hiring! Check Out Our Careers Page For Open Positions:
A Unified eClinical Suite Delivering Real-Time Data
Regulatory Compliance
TrialStat's entire Unified eClinical Suite is developed and deployed with an Agile Methodology and specifically engineered to comply with FDA 21 CFR Part 11 requirements for Electronic Records and Electronic Signatures, HIPAA, GDPR and FISMA. TrialStat maintains a Master Validation Plan and Change Control procedures.
Recent News, Updates & Announcements
Things are happening! Check back often or subscribe to our RSS Feed.
C4 Therapeutics Announces First Patient Dosed in Phase 2 MOMENTUM Trial of Cemsidomide, an Oral IKZF1/3 Degrader, in Combination with Dexamethasone for Relapsed/Refractory Multiple Myeloma
Enrollment for Phase 2 MOMENTUM Trial Expected to Be Completed in Q1 2027 Phase 1b Trial of Cemsidomide in Combination with Elranatamab on Track to Initiate in Q2 2026 Excerpt from the Press Release: WATERTOWN, Mass., Feb. 23, 2026 (GLOBE NEWSWIRE) — C4 Therapeutics, Inc. (C4T) (Nasdaq: CCCC), a clinical-stage biopharmaceutical company dedicated to advancing…
New indication in pediatric patients, including term neonates for Guerbet’s half-dose GBCA, Elucirem™ (Gadopiclenol) Injection
Excerpt from the Press Release: PRINCETON, N.J., Feb. 20, 2026 /PRNewswire/ — Guerbet, a global specialist in contrast media and solutions for medical imaging, is pleased to announce that the U.S. Food and Drug Administration (FDA) has approved a labeling update for Elucirem™ (gadopiclenol) injection. The new indication includes approval for pediatric patients aged 0-2 years…
Daré Bioscience Announces FDA Clearance of IND for Phase 2 Clinical Study of DARE-HPV, a Potential Treatment for Persistent High-Risk HPV Infection, the Most Common Cause of Cervical Cancer
DARE-HPV development supported by $10 million ARPA-H contract; program targets major unmet need with no FDA-approved therapies Excerpt from the Press Release: SAN DIEGO, Feb. 23, 2026 (GLOBE NEWSWIRE) — Daré Bioscience, Inc. (NASDAQ: DARE), a purpose-driven health biotech company solely focused on closing the gap in women’s health between promising science and real-world solutions,…