A Fully Integrated eClinical Platform Designed For CROs
Streamline and simplify your clinical data management with a flexible suite of tools.
Compared to other EDC solutions on the market from MediData, IBM Clinical, Oracle DataTrack, Omnicomm and others, TrialStat is completely unique. TrialStat's eClinical Suite is the most all-encompassing suite of study management tools that can be seamlessly connected to external data sources such as EMR, wearables, and other clinical and non-clinical data and information sources. More importantly, we provide named Project Management and Technical Support, custom pricing models, and custom validated development to provide you with a solution that fit's right in with your expectations.

Comarketing and Joint Business Development Activities
As an eClinical Solutions provider, TrialStat operates withing a broad ecosystem of supporting technologies (CTMS, ETMF, Devices, Safety Systems, other EDC vendors, etc) as well as service providers (CROs, Data Management companies, Regulatory Consultants, Stats, Clinical Consultants, manufacturers, depots, etc.).
As such, we work closely with all of our partners on marketing joint business development activities. As a TrialStat partner you would benefit by participating in joint webinars, industry tradeshows, road shows, and collaborating on projects as well as receiving referrals.
To learn more, please contact us today using the contact form in top right corner, or sending an email to [email protected].
Premium Modules and Capabilities
TrialStat EDC provides integrated IWRS functionality, allowing for any degree of randomization complexity.
Using TrialStat IWRS will enable sites to trigger randomization codes directly without leaving TrialStat EDC, and without the need or added cost of another software system.
Our user-friendly system implements the most current technologies and tools to ensure that your team can successfully randomize patients into your trial without complications or delays.
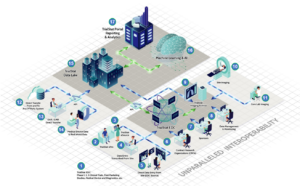
Click the diagram below to explore all of the features of the TrialStat eClinical Suite.
Request Your Demo Today!
From rapid database build through database lock, we deliver consistent quality on-time and on-budget. Ready to upgrade your eClinical toolkit?
Regulatory Compliance
TrialStat's entire Unified eClinical Suite is developed and deployed with an Agile Methodology and specifically engineered to comply with FDA 21 CFR Part 11 requirements for Electronic Records and Electronic Signatures, HIPAA, GDPR and FISMA. TrialStat maintains a Master Validation Plan and Change Control procedures.
A Unified eClinical Suite Delivering Real-Time Data
See TrialStat In Action
Request Your Personalized Demo. Our Clinical Team will organize a tailored demonstration of TrialStat based on your specific study requirements. You'll see first hand how TrialStats unified features, blazing speed, configurable features and comprehensive functionality will help you run more efficient and cost effective studies.