How Does TrialStat Compare To Other EDC Platforms? Book Your Demo Today To Learn More.
Selecting an eClinical Solution shouldn't involve guesswork or crossed fingers.
Can your EDC provider clearly demonstrate what differentiates them in the crowded eClinical technology marketplace? We can.
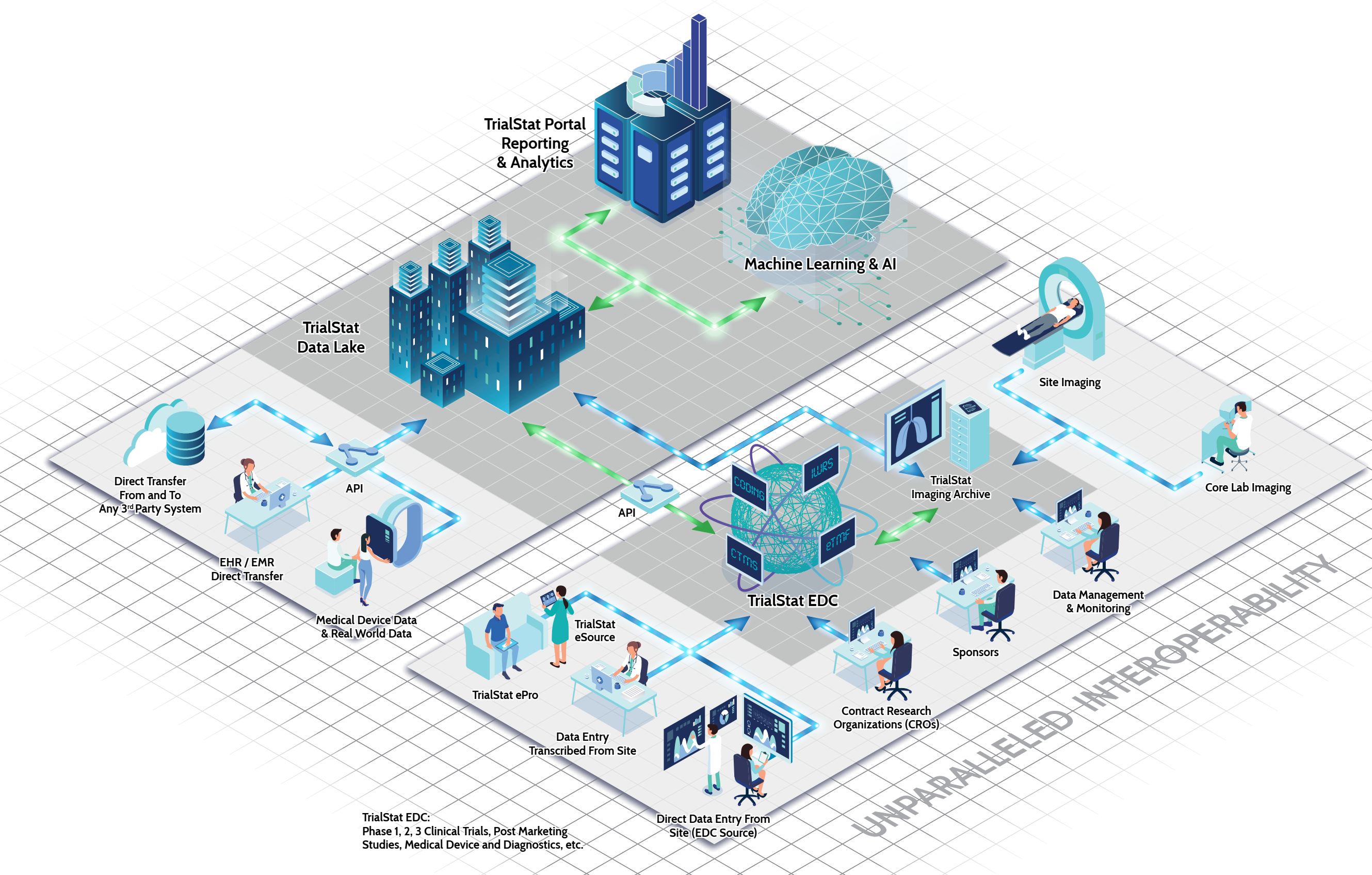
Compared to other EDC solutions on the market, TrialStat is a leader in offering the most all-compassing, flexible suite of study management tools that can also be seamlessly connected to your external data sources such as EMR, wearables, data warehousing, and other clinical and non-clinical data and information sources.
TrialStat’s eClinical Suite consists of a fully unified product offering including EDC, Randomization, ePRO, Adjudication, Coding, AE/SAE Management, Vendor Neutral Image Archive, CTMS and a robust Reporting and Analytics Portal for real-time insights.
Managing multiple vendors and platforms is a huge challenge and increases overall timelines and costs of running your clinical programs. TrialStat offers a single solution that scales easily to match your needs and budgets.
Ready to upgrade your eClinical toolkit?
Book Your Demo Using the Form Below
About TrialStat Solutions Inc.
With more than 15 years of experience, global reach, and clients ranging from diagnostic start-ups to international pharmaceuticals, CROs to consultancies, TrialStat Solutions, Inc. is at the forefront of a new paradigm in life sciences technology, delivering integrated, flexible, and easily implemented on-demand data management and reporting solutions.
Based out of Montreal, Canada, TrialStat is a subsidiary of Jubilant Life Sciences, enabling us to take advantage of cutting edge tools and experts in the AI and Machine Learning spaces and integrate those into our processes, bringing transformational eClinical solutions to our clients that enhance, expedite, and elevate their clinical programs.
See TrialStat In Action
Request Your Personalized Demo. Our Clinical Team will organize a tailored demonstration of TrialStat EDC, TrialStat Portal and TrialStat CTMS based on your specific study requirements. You'll see first hand how the TrialStat unified features, blazing speed, configurable features and comprehensive functionality will help you run more efficient and cost effective studies.