Announcements
Interim safety and pharmacokinetic data from Phase 1 healthy volunteers trial anticipated in 2025 Initiation of the first clinical trial of APG777 and APG990 combination expected to commence in 2025 with the potential for greater efficacy in atopic dermatitis and across I&I diseases Excerpt from the Press Release: SAN FRANCISCO and WALTHAM, Mass., Aug. 19,…
Read MorePreclinical data has demonstrated the potential of SON-1210, the first albumin-binding bifunctional IL-12/IL-15 fusion protein, for solid tumor immunotherapy An Innovative Immuno Oncology Consortium (“IIOC”) led by Oncology experts funded by the Sarcoma Oncology Center will conduct an investigator-initiated Phase 1/2a study of SON-1210 in pancreatic cancer, an indication with significant unmet medical need Company…
Read MoreExcerpt from the Press Release: SAN DIEGO, Aug. 20, 2024 /PRNewswire/ — As the popularity of GLP-1 receptor agonists continues to surge in the treatment of obesity and type 2 diabetes, Evofem Biosciences, Inc. (OTCQB: EVFM) is raising awareness of the potential impact these medications may have on the effectiveness of oral contraceptives. With an…
Read MoreExcerpt from the Press Release: PALO ALTO, Calif., Aug. 22, 2024 /PRNewswire/ — I Peace, a pioneering CDMO in induced pluripotent stem (iPS) cells, and iCamuno Biotherapeutics, a biotech company developing iPS cell-based therapies, today announced a significant milestone with the dosing of the first patient in a clinical trial using iPS cell-derived natural killer (iNK) cells for ovarian…
Read MoreVP-315 was well tolerated with no reported treatment-related serious adverse events All patients treated with VP-315 had a reduction in tumor size with an overall reduction in tumor size of all lesions treated in Part 2 of approximately 86% Approximately 51% of lesions treated in Part 2 achieved complete histological clearance Patients with residual tumor…
Read More– 100% of 9 treated BCCs (1 superficial, 4 nodular, and 4 infiltrating) across 7 enrolled subjects to date demonstrate prompt, visible, and robust tumor responses during treatment with SM-020 1% BID for 28 days, resulting in partial or total reduction of clinically visible tumor. Excerpt from the Press Release: BOSTON–(BUSINESS WIRE)–DermBiont, a clinical-stage biotechnology…
Read MoreExcerpt from the Press Release: TÜBINGEN, GERMANY and BOSTON, MA / ACCESSWIRE / August 15, 2024 / CureVac N.V. (Nasdaq:CVAC) (“CureVac”), a global biopharmaceutical company developing a new class of transformative medicines based on messenger ribonucleic acid (“mRNA”), today announced the start of the dose-confirmation Part B of its ongoing Phase 1 study in patients…
Read MoreEquity investment reflects commitment to Treg science and support of Abata’s approach and programs Excerpt from the Press Release: WATERTOWN, Mass., Aug. 15, 2024 (GLOBE NEWSWIRE) — Abata Therapeutics, a company focused on transforming lives with Treg therapies for severe autoimmune and inflammatory diseases, today announced it has received an equity investment from Bristol Myers…
Read MoreExcerpt from the Press Release: SAN DIEGO, Aug. 14, 2024 (GLOBE NEWSWIRE) — Turnstone Biologics Corp. (“Turnstone” or the “Company”) (Nasdaq: TSBX), a clinical-stage biotechnology company developing a differentiated approach to treat and cure patients with solid tumors by pioneering selected tumor-infiltrating lymphocyte (Selected TIL) therapy, today reported positive initial data from its Phase 1…
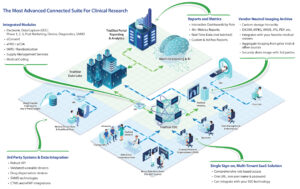
Read MoreTrialStat’s comprehensive Electronic Data Capture (EDC) Platform integrated with our Vendor Neutral Imaging Archive (VNA) and Medical Imaging viewers is the right solution for your trials! Image Upload from EDC to VNA with de-identification Integrated with your Medical Viewers Secure, remote access to the VNA for Radiologist access with their preferred medical imaging viewer Long term…
Read More