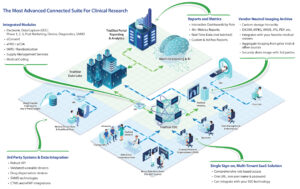
eClinical Technology
Excerpt from the Press Release: SAN DIEGO, July 2, 2025 /PRNewswire/ — A new prospective study published in BMC Cancer demonstrates that a novel blood-based multi-omics test, SeekInClarity, can accurately assess and predict therapeutic outcomes in lymphoma patients across different subtypes—potentially transforming how clinicians monitor treatment response. Study Highlights Clinical Impact Current standards for monitoring lymphoma…
Read MoreIn the complex world of pharmaceutical clinical trials, efficiency, accuracy, and real-time data access are paramount. Systems integration plays a crucial role in ensuring these elements are met, significantly improving the trial process. By integrating multiple technology systems-such as Electronic Data Capture (EDC), clinical trial management systems (CTMS), and imaging platforms-trial teams can create a…
Read MoreCollaboration utilizes PeriNess’ expertise in clinical development of human recombinant DNase and bolsters efforts towards clinical proof-of-concept studies in multiple indications Excerpt from the Press Release: FRAMINGHAM, MA / ACCESSWIRE / December 5, 2024 / Xenetic Biosciences, Inc. (NASDAQ:XBIO) (“Xenetic” or the “Company”), a biopharmaceutical company focused on advancing innovative immuno-oncology technologies addressing hard to…
Read MoreTrialStat’s comprehensive Electronic Data Capture (EDC) Platform integrated with our Vendor Neutral Imaging Archive (VNA) and Medical Imaging viewers is the right solution for your trials! Image Upload from EDC to VNA with de-identification Integrated with your Medical Viewers Secure, remote access to the VNA for Radiologist access with their preferred medical imaging viewer Long term…
Read MoreSelecting the Right EDC: A Critical Decision for Clinical Trial Success In the ever-evolving landscape of clinical trials, the choice of an Electronic Data Capture (EDC) technology vendor is more than a mere procedural step; it’s a pivotal decision that can significantly impact the success of your clinical research. Whether you’re embarking on your first…
Read More