Search Results: 100% Pass Quiz High Pass-Rate Juniper - JN0-281 - Data Center, Associate (JNCIA-DC) New Braindumps Ebook 🐍 Open ➽ www.pdfvce.com 🢪 enter ➡ JN0-281 ️⬅️ and obtain a free download 🔮Valid Exam JN0-281 Book
Excerpt from the Press Release: BOSTON, July 15, 2021 /PRNewswire/ — COTA, Inc., an oncology real-world data and analytics company, announced a new research partnership with MedStar Health. COTA will support MedStar Health in its use of real-world data to accelerate scientific discovery and improve care for cancer patients. MedStar Health is the largest healthcare provider in the Maryland and Washington, D.C.,…
Read MoreExcerpt from the Press Release: EMERYVILLE, Calif.–(BUSINESS WIRE)–Nutcracker Therapeutics, Inc., a biotechnology company dedicated to developing transformative RNA therapies through its proprietary technology platform, recently presented two posters at the American Association for Cancer Research (AACR) Annual Meeting in San Diego: one showcasing the latest preclinical data for the company’s mRNA drug candidate for prostate…
Read More– Key Biomarker Activity Confirmed for Evaluation in Upcoming Phase 1 Study – Excerpt from the Press Release: SAN DIEGO–(BUSINESS WIRE)–Revelation Biosciences Inc. (NASDAQ: REVB) (the “Company” or “Revelation”), announced today that data from recent preclinical studies of the Company’s Gemini formulation demonstrated its therapeutic potential as a preventative therapy across multiple indications including infection…
Read MoreRP-1664 is a Potential First-in-Class, Selective, PLK4 Inhibitor Excerpt from the Press Release: CAMBRIDGE, Mass. & MONTREAL–(BUSINESS WIRE)–Repare Therapeutics Inc. (“Repare” or the “Company”) (Nasdaq: RPTX), a leading clinical-stage precision oncology company, today announced the first patient has been dosed in the Company’s Phase 1 LIONS (PLK4 Inhibitor in Advanced Solid Tumors) clinical trial evaluating…
Read MoreAbivertinib is an oral capsule (100 mg QD or two 50-mg capsules a day) that potentially reduces cytokine storm associated with acute respiratory distress syndrome (ARDS) in severe hospitalized COVID-19 patients. Preliminary results from two completed Phase 2 studies: US study (N=96) and Brazil study (N=400) have identified an At-Risk COVID-19 Patient Population – Hospitalized COVID patients receiving oxygen support by…
Read MoreData published in the Journal of Burn Care & Research show 83% of patients with posterior burns (mean total body surface area of 56%) had successful engraftment of Epicel after one or two applications 90% survival rate following Epicel and/or Epicel plus split thickness skin graft Study demonstrates that standardized treatment protocols and surgical plans for…
Read MoreGPH101, now called nulabeglogene autogedtemcel (nula-cel), designed to directly correct the genetic mutation that causes sickle cell disease Initial proof-of-concept data from Phase 1/2 CEDAR trial anticipated in mid-2023 Excerpt from the Press Release: SOUTH SAN FRANCISCO, Calif.–(BUSINESS WIRE)–Graphite Bio, Inc. (Nasdaq: GRPH), a clinical-stage, next-generation gene editing company harnessing the power of high-efficiency precision…
Read MoreLogica, an offering from Charles River and Valo Health, will be used to discover first-in-class therapeutics against high value targets in the Related Sciences portfolio Excerpt from the Press Release: WILMINGTON, Mass. & DENVER–(BUSINESS WIRE)–Charles River Laboratories International, Inc. (NYSE: CRL) and Related Sciences (“RS”), a data science-driven drug discovery firm, today announced a multi-program…
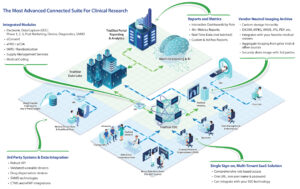
Read MoreTrialStat’s comprehensive Electronic Data Capture (EDC) Platform integrated with our Vendor Neutral Imaging Archive (VNA) and Medical Imaging viewers is the right solution for your trials! Image Upload from EDC to VNA with de-identification Integrated with your Medical Viewers Secure, remote access to the VNA for Radiologist access with their preferred medical imaging viewer Long term…
Read MoreEasily Migrate from edc2go! Trust TrialStat to Migrate Your Study from edc2go! Don’t be held hostage by your CRO or EDC Vendor. Take ownership of your study data and benefit from a Fully Unified eClinical Suite. With the discontinuation of edc2go, now is the time to rethink which Electronic Data Capture Vendor and Platform to…
Read More