Announcements
Christopher Kata and the TrialStat team are exhibiting at this years DPHARM meeting on September 20th through the 22nd in booth #57. If you’re attending be sure to to reach out to Christopher to schedule a one on meeting or just drop by booth #57 at your convenience! We’re looking forward to providing live, personalized…
Read MoreExcerpt from the Press Release: MOUNTAIN VIEW, Calif.–(BUSINESS WIRE)–Two-year results from FISH&CHIPS, a real world, multi-center, retrospective clinical study with 90,553 patients conducted by the National Health Service (NHS) England were presented at the 2023 European Society of Cardiology (ESC) Congress meeting in Amsterdam by Principal Investigator, Dr. Tim Fairbairn, Consultant Cardiologist, Liverpool Heart and Chest Hospital. FFRCT (CCTA-derived Fractional…
Read MoreAnti-tumor activity in patients with NRAS Q61X melanoma and KRAS Q61X NSCLC supports tissue agnostic development in RAS Q61X solid tumors Initial Phase 1b combination data from SEACRAFT-1 expected in Q2-Q4 2024 Dosing of first patient in pivotal SEACRAFT-2 trial in NRAS-mutant melanoma expected in H1 2024 Excerpt from the Press Release: SAN DIEGO, Aug.…
Read MoreExcerpt from the Press Release: SOUTH SAN FRANCISCO, Calif., Aug. 30, 2023 (GLOBE NEWSWIRE) — Denali Therapeutics Inc. (NASDAQ: DNLI), a biopharmaceutical company developing a broad portfolio of product candidates engineered to cross the blood-brain barrier for the treatment of neurodegenerative diseases and lysosomal storage diseases, today announced new interim data from the ongoing open-label,…
Read MoreExcerpt from the Press Release: CAMBRIDGE, Mass., Aug. 31, 2023 (GLOBE NEWSWIRE) — Foghorn® Therapeutics Inc. (Nasdaq: FHTX), a clinical-stage biotechnology company pioneering a new class of medicines that treat serious disease by correcting abnormal gene expression, today announced the first patient has been dosed in the Phase 1 study of FHD-286 in combination with decitabine…
Read MoreExcerpt from the Press Release: BOSTON–(BUSINESS WIRE)–Astria Therapeutics, Inc. (NASDAQ:ATXS), a biopharmaceutical company developing STAR-0215 for the treatment of hereditary angioedema (HAE) and focused on life-changing therapies for rare and niche allergic and immunological diseases, today announced that preclinical data supporting STAR-0215’s profile as a potential long-acting therapy for hereditary angioedema (HAE) dosed once every three…
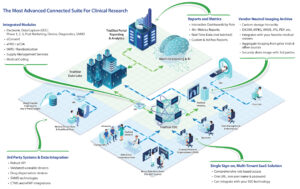
Read MoreYou should not have to compromise your study design simply because your eClinical Technology does not support your unique study requirements. At TrialStat we do things differently. We believe that you should not have to compromise and to make that a reality, we specialize in Custom Validated Development. Whether it’s a small change in workflow,…
Read More– CABINET trial will be unblinded and stopped early due to a dramatic improvement in efficacy per a unanimous recommendation by The Alliance for Clinical Trials in Oncology independent Data and Safety Monitoring Board – – Based on positive results, findings will be discussed with the U.S. Food and Drug Administration – Excerpt from the…
Read MoreExcerpt from the Press Release: SAN FRANCISCO, Aug. 25, 2023 /PRNewswire/ — AusperBio Therapeutics, Inc. and Ausper Biopharma Co., Ltd. (Together AusperBio), a clinical-stage biotech company committed to advancing antiviral therapies and vaccines, with a primary focus on achieving a functional cure for chronic hepatitis B (CHB) infection, today announced the U.S. Food and Drug…
Read More– Positive Phase 2 EXACT Trial results at 6-months underscore significant potential of investigational therapy in refractory angina – Six-month data have since been sustained out to 12-months supporting durability of XC001 safety and efficacy profile – XC001 targets unmet medical need among patients with refractory angina who have a debilitating quality-of-life burden and no…
Read More