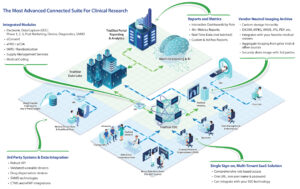
eClinical Technology
You should not have to compromise your study design simply because your eClinical Technology does not support your unique study requirements. At TrialStat we do things differently. We believe that you should not have to compromise and to make that a reality, we specialize in Custom Validated Development. Whether it’s a small change in workflow,…
Read MoreTrialStat’s eConsent platform was designed and developed to solve the most pressing trial enrolment and protocol compliance problems. With a completely customizable workflow, content templates, multimedia capabilities, and multilingual options, Sponsors and CROs have the tools they need to create an immersive eConsent process for trial participants. Our eConsent module is fully integrated with our…
Read MoreThe landscape of clinical research is constantly evolving, and businesses need to keep up with the latest technology to remain competitive. However, investing in eClinical technology is not just about finding a solution for current needs, it’s equally important to think ahead and ensure that your technology investment can adapt and scale to meet future…
Read MoreFor 2023, we’re taking our blog in a new direction with more content directly from our team, more detailed information for our readers answering common questions we get, and helpful expert information and ideas you can use. We hope you enjoy this new approach. For this week, we’re covering an introduction (or refresher) to TrialStat…
Read More— Polymer-conjugated IL-15 (NKTR-255) induced CD8+ T cell and natural killer cell proliferation in vivo — — NKTR-255 enhanced the efficacy of human CD19 CAR-T cells in a xenogeneic lymphoma model — Excerpt from the Press Release: SAN FRANCISCO, Nov. 9, 2022 /PRNewswire/ — Nektar Therapeutics (Nasdaq: NKTR) today announced the publication of preclinical data…
Read MoreA tool for reconstruction of a 3D model of the knee from 2D X-ray images is being evaluated on clinical data at a leading medical center with promising results Excerpt from the Press Release: TEL AVIV, Israel and SAN JOSE, Calif., Oct. 27, 2022 /PRNewswire/ — RSIP Vision, an experienced developer of groundbreaking AI technologies for…
Read MoreExcerpt from the Press Release: LOS ALTOS, Calif., Sept. 22, 2022 /PRNewswire/ — Efemoral Medical, developer of advanced interventional bioresorbable therapies, announced today that it has been awarded a Phase II Small Business Innovation Research (SBIR) grant by the National Institutes of Health. SBIR grants are intended to stimulate technological innovation and encourage small United…
Read MoreThree-year, standalone (not combined with cataract surgery) data shows the non-implantable minimally invasive glaucoma surgery effectively reduces intraocular pressure and reduces need for IOP-lowering medications in open-angle glaucoma patients Excerpt from the Press Release: MENLO PARK, Calif., Aug. 16, 2022 (GLOBE NEWSWIRE) — Sight Sciences, Inc. (Nasdaq: SGHT), an eyecare technology company focused on developing…
Read MoreTreatment with epigenomic controller, OTX-2002, resulted in robust in vivo efficacy in xenograft tumor models OTX-2002 successfully achieved pre-transcriptional downregulation of hepatocyte MYC gene expression in non-human primates Clinical potential of OTX-2002 as a monotherapy or in combination with existing standard-of-care therapies, including immune checkpoint inhibitors IND filed by the company to advance OTX-2002 into…
Read More- « Previous
- 1
- 2
- 3
- Next »